Located in Group 9 and Period 4 of the Periodic Table is the element nowadays used to produce rechargeable batteries. This is Cobalt.
First discovered and first isolated by Georg Brandt in 1735.
 |
| Georg Brandt |
 |
| Dissertatio de Semi-Metallis |
new chemical element, a semi-metal, which he named cobalt. ... Brandt discovered the new metal in
an ore German miners called kobold. The element name cobalt comes from the German 'kobold'
meaning goblin. This element is just to cure some types of cancer.
Cobalt is also used to make alloys for jet engines and gas turbines, magnetic steels and some types of
stainless steels. Cobalt-60, a radioactive isotope of cobalt, is an important source of gamma rays and is
used to treat some forms of cancer and as a medical tracer.
 |
| Plane Engine |
Cobalt was the first metal to be discovered since prehistoric times and the first metal with a
recorded discoverer.
Too much or too little cobalt in the body can cause health issues.
 |
| Asthma |
breath and dyspnea to decreased pulmonary function, nodular fibrosis, permanent disability, and
death. Exposure to cobalt may cause weight loss, dermatitis, and respiratory hypersensitivity.
Name : Cobalt
German Name : Kobalt
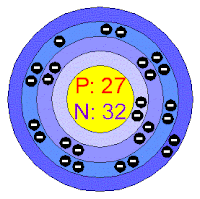
Electrons per shell : [2, 8, 15, 2]
Discoverer : Georg Brandt
Isolator : Georg Brandt
Element's : Atomic Mass : 58.933 u
: Density : 8.90 g/cm3
: Density : 8.90 g/cm3
: Type : Transition Metal
Chemical Properties :
- Relatively reactive metal
- Able to react with most acids to produce hydrogen gas
- Does not react with water that is at room temperature
- Corrosion Resistant
- Combines with oxygen in the air
- Catch on fire and burn when in the form of powder and reacting with oxygen
- 40 isotopes : 3 of them are :
Cobalt - 56 : Protons : 27
: Electrons : 27
( Half Life : 77.233 days)
Cobalt - 57 : Protons : 27
: Neutrons : 30
: Electrons : 27
( Half Life : 271.74 days)
Cobalt - 58 : Protons : 27
: Neutrons : 31
: Electrons : 27
( Half Life : 70.86 days)
Physical Properties :
- Silvery, bluish-gray
- Brittle
- Conductor of heat
- Conductor of electricity
- Lustrous
- Hard
- Magnetic
- Melting point : 1495 degrees Celsius ( 2723 Fahrenheit )
- Boiling point : 2870 degrees Celsius ( 5198 Fahrenheit )
How Cobalt got it's name?
Cobalt got the name because cobalt was responsible for the horrible and mysterious deaths of
miners. 'Kobalt' , which means goblin.
miners. 'Kobalt' , which means goblin.
 |
| Goblin |
Cobalt is used in electroplating(usually green and blue in colour) due to its hardness, attractive appearance and resistance to oxidation. Globally, cobalt is used in rechargeable batteries to help increase the battery life, stability and to reduce corrosion. Superalloys that are cobalt-based are used
in jet engines because of their stability at high temperatures.
Cobalt salts are used to impart blue and green colours in ceramics and glasses. It is also used as an
indicator for water in desiccants. Other than that, cobalt is widely used in radiotherapy and as a tracer.
Cobalt-60 is a synthetic radioactive isotope of cobalt produced artificially in nuclear reactors. It
produces gamma ray and is used in cancer treatments.
in jet engines because of their stability at high temperatures.
 |
| Cobalt superalloy |
Cobalt salts are used to impart blue and green colours in ceramics and glasses. It is also used as an
indicator for water in desiccants. Other than that, cobalt is widely used in radiotherapy and as a tracer.
Cobalt-60 is a synthetic radioactive isotope of cobalt produced artificially in nuclear reactors. It
produces gamma ray and is used in cancer treatments.
 |
| Cobalt chloride |
THIS IS THE END OF COBALT
To go to homepage, click this link :
To go to Chemistry Element Page, click this link :
Thanks for visiting.
If there is any improvements needed, feel free to comment at the comment section below.
Thank you.